Micro Eye Drops
mu-Drop supplies components and dedicated equipment to blood banks, hospitals and the pharmaceutical industry to manufacture micro eye drops.
- Ten times smaller eye drops
- Less API required
- Three component eye drop applicator
- Dedicated assembly equipment (CE certified)
- Efficient manufacturing process
- MDR compliant documentation
- mu-Drop is ISO-13485:2016 certified (mu-Drop ISO-13485 certificate)
- Patented
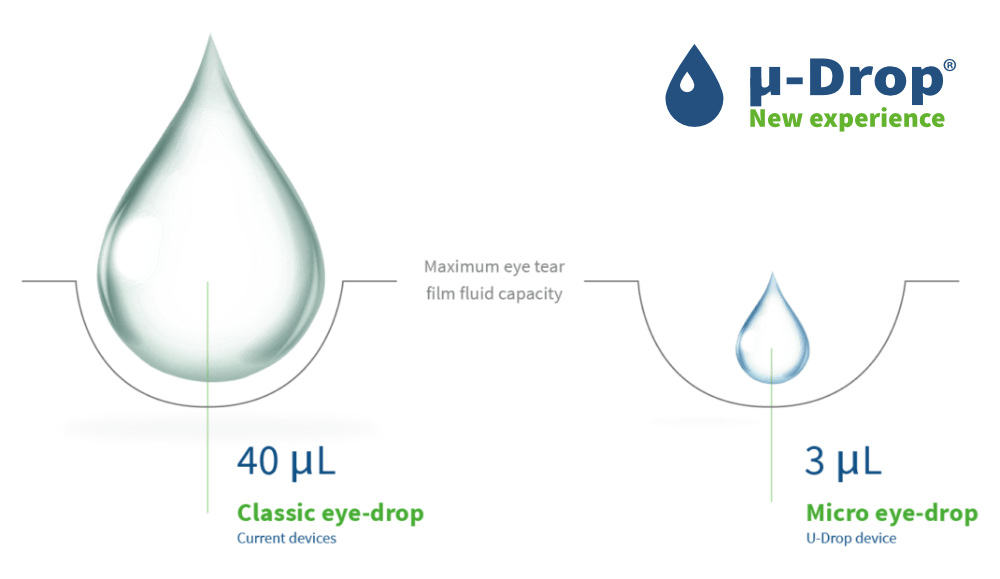
mu-Drop micro drops fit in the eye
- 10 times smaller than classic eye drops
- Fit in the eye
- No spillage
- Comfortable
- Simple to use
mu-Drop micro eye drops fit in the eye which may reduce side effects, improve quality of life and increase ophthalmic therapeutic effectiveness.
- Single use
- No preservatives
- Effective
- Well tolerated
- Effective
Patient therapy adherence is the major challenge for eye drop treatment. Excess eye drop medication may cause very annoying side effects for patients;
- Secondary Dry Eye Syndrome
- Systemic side effects
- Local side effects
No excess medication is administered by mu-Drop micro eye drops and therefore is the tolerability very well, secondary Dry Eye Syndrome avoided and systemic and local side effects absent. mu-Drop micro eye drops drive product quality.
- Versatile, scalable, robust manufacturing technology
- Cost effective
- Improved product integrity
- Preservative free
