Flow Neuroscience
Treat Depression.
Differently.
What is Flow?
Flow is a depression treatment that uses a tDCS headset and an in-app behavioural therapy programme.
The Flow tDCS is a Class IIa medical device approved for the treatment of major depressive disorder (MDD) in adults, either as monotherapy or as an adjunctive treatment. The device will be accessible to patients under the supervision of an appropriately qualified health practitioner and able to be ordered through the Patient Purchase Portal on Aurora Direct.
It is important to always use Flow in accordance with the Flow Neuroscience User Manual.

Maximise Treatment Outcomes.
Minimise Treatment Barriers.
Provide your patients with an effective1 and easy-to-use, at-home depression treatment option.
Flow has a favourable side effect profile1,2 and can be used as a standalone intervention or in combination with other treatment approaches including medication.
Join more than 500 private practice clinics and 5 NHS trusts and build Flow into your depression treatment pathway.
Flow is designed to optimise outcomes for both patients and clinicians by offering tailored solutions for each. Patients are equipped with the Flow tDCS Headset and companion app, allowing them to conveniently manage their treatment from home. Clinicians benefit from a remote monitoring platform that provides real-time insights into patient adherence, outcomes, and supports data-driven care decisions. Clinicians can adjust and customise patient stimulation schedules according to clinical need*.
*Subject to clinical qualifications and training
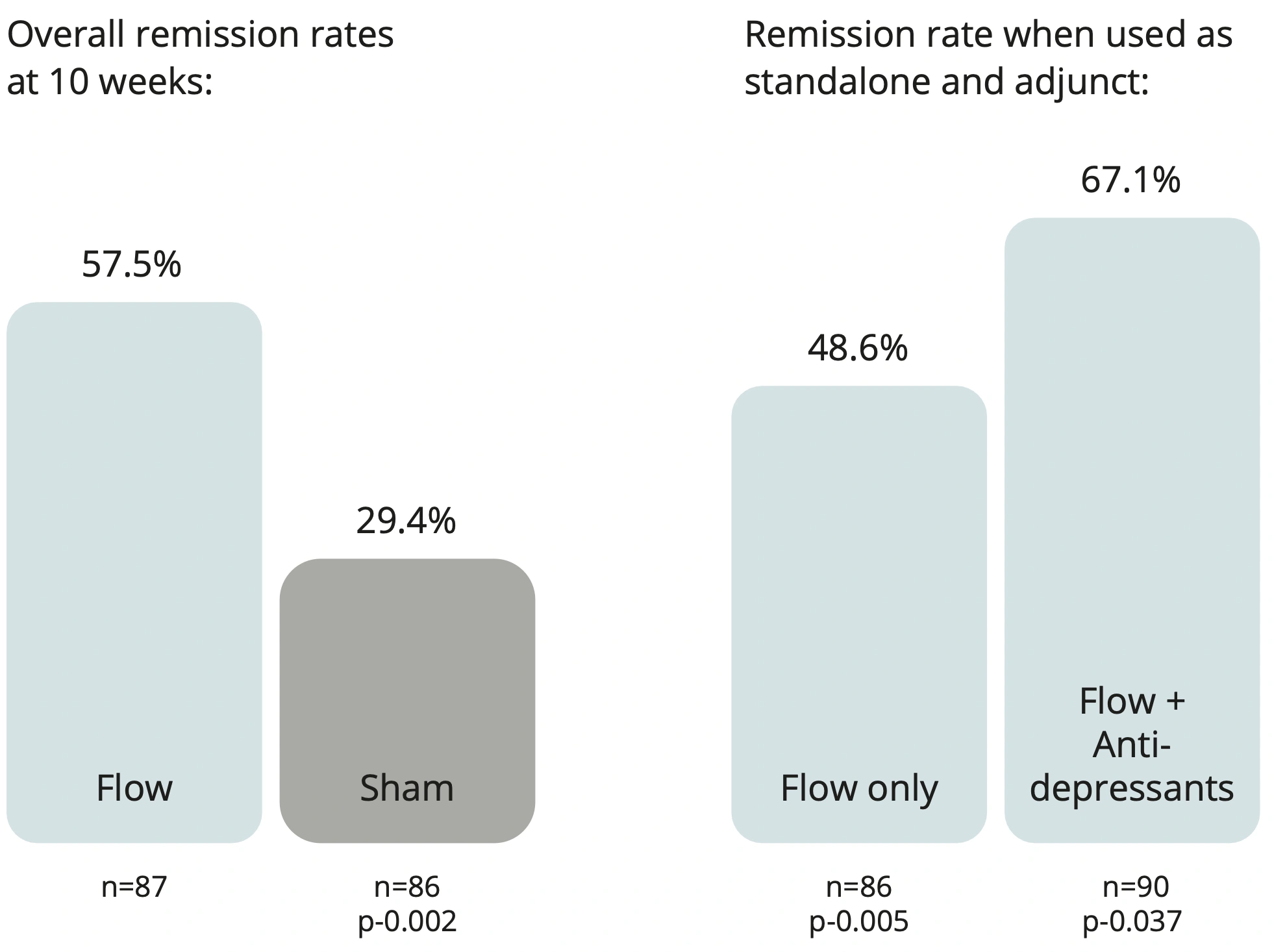
57.5% of study participants achieved remission in 10 weeks*
The recent EMPOWER clinical trial results, published in Nature Medicine, demonstrate Flow is effective and superior to the sham treatment1. This 10-week double-blind placebo-controlled randomised study reviewed the use of Flow compared to placebo across 173 adult participants diagnosed with unipolar depression (moderate to severe).
Real-world data2 and various ongoing pilots3 show similar results.
The EMPOWER trial also highlighted a potential amplification when Flow was used in combination with an antidepressant. This impact has also been reported in real-world cases.
Could Flow be of interest to your patients looking for depression relief?
*Remission defined as a score of ≤12 on MADRS

Flow in practice

Over 30,000 real-world Flow users and 900,000 Flow stimulation sessions completed

300+ clinics offering Flow globally
Who is right for Flow?
Flow is approved for use in anyone 18 or older with a Major Depressive Disorder (MDD) disorder, either as monotherapy or as an adjunctive treatment. The device is only to be used under the supervision of an appropriately qualified health practitioner.
Flow has been provided to patients across Primary Care, Perinatal Services, Crisis Services and Community Mental Health settings internationally.
Results at 10 weeks from a multisite, double-blind placebo-controlled randomised superiority trial were statistically significant for both primary and secondary endpoints. Flow achieved a 57.5% remission rate using the MADRS scale and was effective as both standalone and adjunctive therapy*.
Australian News
Mechanism of action
The Flow headset delivers a gentle 2mA electric current (transcranial Direct Current Stimulation, tDCS) to the left dorsolateral prefrontal cortex.
This region is crucial for emotional regulation and is often hypoactive in individuals with depression. tDCS enhances neuronal activity in this area, counteracting hypoactivity, improving mood and emotional regulation.
Track and optimise patient care with the Flow Clinician Platform
Effortlessly review your patients’ adherence and progress with Flow’s Clinician Platform. Accessible anytime, anywhere through a secure web browser, this platform aggregates data including weekly MADRS-s scores and daily stimulation tracking. With these insights, you can monitor patient response, ensure treatment adherence, and easily customise individual treatment schedules to help optimise patient outcomes.

Open-label patient cohort study in an NHS primary care
general practice found at 6 weeks: 2
- 58.1% of patients showed reliable improvement, and 32.3% achieved remission using the PHQ-9
- Significant improvements were observed in functioning (WSAS) and health-related quality of life (EQ-5D-5L)
Improvements in Depression Severity and Functional Impairment
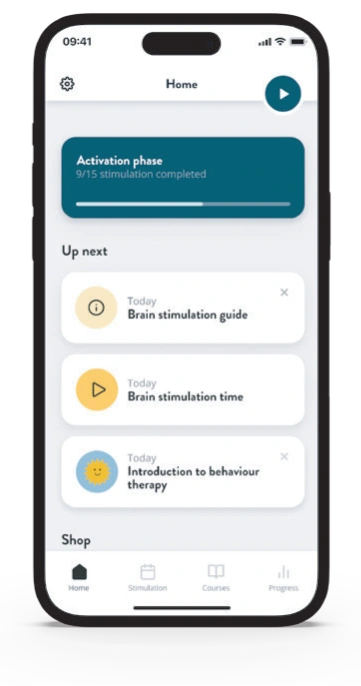
The Flow Treatment Schedule
The standard treatment is split into 2 phases – activation and strengthening. Each session (termed stimulation) is 30 minutes long. Patients complete a MADRS-s survey at the beginning of each week to monitor progress.

Activation Phase
Weeks 1-3
- Includes 5 stimulations a week
- Introduces optional behavioural therapy courses via the app
Strengthening Phase
Week 4 onwards
- Includes up to 2 stimulations a week
- Continues to strengthen and preserve results
- Continue behavioural therapy courses
Behavioural therapy
The Flow app includes 7 behavioural therapy courses including behavioural activation, exercise, meditation, diet, and sleep hygiene, totalling over 50 short therapy sessions. These courses are entirely optional and do not interfere with the Flow stimulation protocol.
Contraindications
No universal contraindications.
Warnings and Precautions
- Broken/inflamed/infected skin (including, for example, psoriasis) at the electrode site
- Cranial or intracranial implant (e.g. brain clips, deep brain stimulators)
- Craniofacial abnormalities (e.g., congenital deformities, severe trauma, or reconstructive surgeries) which may affect electrode placement
- Epilepsy or history of seizures
- Active suicidal ideation (requires closer monitoring)
- History of hypomania or mania (may require closer monitoring)
For additional information around warnings and precautions please refer to User Manual.
Special populations
Other chronic conditions: No known interactions with medications; used by individuals with diabetes, heart disease, hypertension, asthma, co- occurring mental health conditions, neurodivergence, and brain injuries/ disorders without reported safety concerns.
Pregnancy: Not licensed for use; no safety concerns identified in existing studies, research ongoing.
Breastfeeding: Safe to use; no effect on breast milk production.
Postpartum: Appears safe for postpartum depression and successfully used in NHS pilots.
Children and Adolescents: Not licensed for use; studies ongoing for ages 14+
Clinical review and customisation
Review your patients’ progress and adherence through the Flow Clinician Platform (CPP). Accessible anytime and anywhere through a secure web browser, this platform streamlines patient visits by providing informed insights. It also allows for the customisation of treatment protocols (maximum 1 per day, 7 per week) based on clinical judgement to address individual patient needs. This setup ensures personalised treatment adjustments to help optimise patient outcomes.
The suggested initial treatment duration is 10 weeks. Following 10 weeks, patients’ response should be assessed and length of continued treatment determined, taking into consideration the effectiveness to date and the risk of relapse.
Flow Research

Transcranial Direct Current stimulator (tDCS) has been used in clinics for over 25 years to treat depression. There are multiple sources of evidence that show it is effective and there are no serious side effects. These range from placebo-controlled clinical trials, the National Health System (NHS) in the United Kingdom studies and open label trials. We have gathered the most prominent research papers in the tDCS community and listed them below.
Placebo-controlled randomised clinical trial
Published in the authoritative Nature Medicine, the design of this trial is considered the gold standard method for evaluating efficacy and took place at Kings College London, UTHealth Houston and the University of East London.
The clinical trial, which lasted 10 weeks, was the largest of its kind and involved patients in the US and UK. Results showed that 57.5% of patients in the treatment group went into remission (meaning that they are no longer considered to be depressed), whilst 64.2% were measured to have an improvement in symptoms of at least 50%. Patients who took a 10-week course of the treatment were about twice as likely to see their depression go into remission than those in a control group who performed the same procedure with the current switched off.
No serious side effects associated with using the device were reported from the treatment group.
Woodham, R.D., Selvaraj, S., Lajmi, N. et al October 2024
“Beneficial effects in cases of depression that doesn’t respond to drugs or therapy.” →
Independently published NHS studies
5 NHS services have been using Flow, they each have independently published their results.
Crisis Team, NHS Leicestershire – October 2024
80% of patients reported a decrease in their depression symptoms →
Community Mental Health, NHS Northamptonshire – February 2024
Flow has been successfully integrated into a CMHT depression treatment →
Postnatal depression, NHS Northamptonshire – August 2024
Improvements in depressive symptoms, mood, sleep quality, and overall well-being →
GP Services, NHS Northamptonshire – November 2023
Most participants described a positive impact on depressive symptoms, sleep, and functioning →
GP Services, NHS Northamptonshire – May 2024
Flow tDCS can be delivered through a primary healthcare general practice service →
Clinical research into tDCS
The Flow brain stimulation treatment is based on decades of clinical research with the most recent meta-analyses showing that tDCS has similar efficacy to other treatments but with fewer side effects.
Across 20+ randomised controlled trials, tDCS has been shown to be superior to placebo/sham and no serious adverse events have been observed. tDCS provides an alternative to medication that is effective, safe and accessible.
Nikolin et al. March 2023
tDCS effect sizes reached a peak at around 6 weeks →
Frengi et al. July 2020
tDCS for depression is definitely effective (level A evidence) →
Moffa et al. April 2020
A meta analysis: tDCS was an effective intervention at reducing depressive symptoms →
Razza et al. February 2020
An analysis of 23 clinical trials with 1092 patients, showing that tDCS is superior to placebo/sham →
Chhabara et al February 2020
Side effects from tDCS are mild, transient and well tolerated →
Sharafi et al. July 2019
tDCS is effective in cases where antidepressants don’t work →
Wang et al. June 2019
A meta-analysis; the intervention of tDCS was superior to the use of sham tDCS →
Mutz et al. March 2019
tDCS was found to be efficacious and comparable to TMS →
Pavlova et al. March 2018
30-minute tDCS sessions lead to better outcomes than 20-minute sessions →
Bikson et al. Feb 2018
Low-energy brain stimulation is safe to use →
Brunoni et al. June 2017
Study including 245 patients, comparing tDCS with a common antidepressant →
Bikson et al. June 2016
No serious adverse effects were found across 33,000 tDCS sessions with 1000 users →
Valiengo et al. July 2013
tDCS is a safe long-term follow-up treatment →
Brunoni et al. April 2013
Combining tDCS and a common antidepressant increases the efficacy of each treatment →
Loo et al. January 2012
tDCS is safe and effective for treating depression →
Support and contact Information
For technical support and assistance, patients can contact info@aurorabioscience.com.au
Clinicians can enquire about setup, pricing, and training support by contacting info@aurorabioscience.com.au
Adverse events should be reported to Aurora BioScience Pty Ltd by emailing info@aurorabioscience.com.au
References:
- *Woodham, R.D., Selvaraj, S., Lajmi, N. et al. Nat Med (2024).
- Data on file. Flow Neuroscience Real-world reports.
- Griffiths, C. et al. (2024). Open Journal of Depression. 13(02), 25–39.
- Woodham, R. D. et al. (2023). Psychiatry Research; 284.
ARTG No. 509725 and 510555 Aurora BioScience Pty Ltd is the Australian TGA sponsor for Flow Neuroscience. The Aurora Direct Online Store belongs to and is part of the operations of Aurora BioScience PTY Ltd. Flow – Transcranial Direct Current stimulator (tDCS) is only to be used under the supervision of an appropriately qualified health practitioner.
