Chemo Mouthpiece™

Reliable and Effective Oral Cryotherapy
For The Therapeutic Management of Oral Mucositis
- Oral Mucositis is a condition that causes painful swelling and mouth sores.
- Chemo Mouthpiece™ is a safe and effective cold therapy method used for the reduction of pain, inflammation and swelling
- Excellent alternative to ice chips

Intraoral Ice Pack
Chemotherapy has many painful and unpleasant side effects, among which is oral mucositis (mouth sores).
The most common preventative method used in both hospitals and infusion centres worldwide is oral cryotherapy, which entails using ice chips to cool the gums prior to and during infusion. However, ice chips are imperfect. The oral cavity isn’t cooled entirely, they are inefficient and are not always tolerated by every patient. People who have sensitive teeth may struggle with ice chips and patients who require oxygen cannot use them at all.
There is now a simple solution, the patent-pending Chemo Mouthpiece™ Intraoral scanning has shown CMP cools the patient’s gums, tongue, and pallet (the entire oral cavity) far more effectively than ice chips.^

Features and Benefits:
- Cools the entire oral cavity
- Stays frozen for 30+ minutes*
- No Rx required
- Single patient, multi-use
- One size fits most adults
- Can be used by those with sensitive teeth
- Can be used by those who require oxygen
- Made with a proprietary liquid formulation and FDA medical grade silicone
- Easily cleaned with hot water and soap
Intraoral scanning has shown Chemo Mouthpiece™ cools a patient’s gums, tongue, and pallet (entire oral cavity) far more effectively than ice chips, see below. The oral cavity is cooled uniformly and the mouthpiece stays cold for 30+ minutes.
On average, Chemo Mouthpiece™ lowers the oral cavity temperature by 17.4 ºC.
*Starting temp. is an average temp. of the gums, tongue, and pallet
**Ending temp. is an average temp. of the gums, tongue, and pallet
-
Temperature Log After 30 Minutes (ºC)
Subject #
Oral Cavity Start Temp*
Oral Cavity End Temp**
Difference
1
31.8º
18.9º
12.9º
2
34.4º
16.7º
17.7º
3
34.3º
18º
16.3º
4
35.7º
14.5º
21.2º
5
35.4º
20.7º
14.7º
6
35.5º
14.9º
20.6º
7
34.1º
17.8º
16.3º
8
35.1º
19.6º
15.5º
9
34.8º
18.7º
16.1º
10
34.9º
16.2º
18.7º
11
34.2º
13.2º
21.0º
12
35º
17.7º
17.3º
Average:
34.6º
17.2º
17.4º
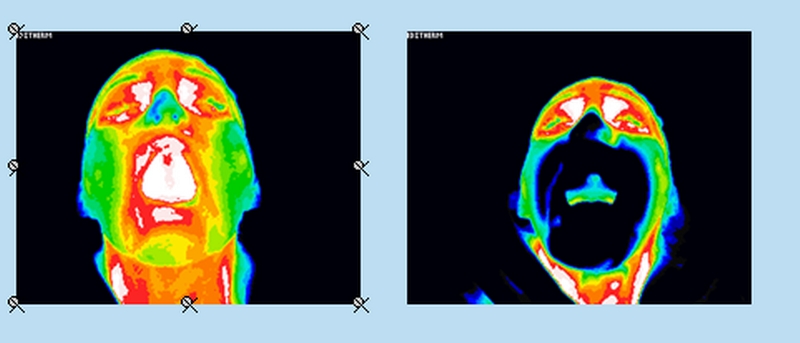
^Intraoral Scanning Results
This diagram shows the subject’s heat distribution before the Chemo Mouthpiece™ was administered. Right shows the heat distribution afterwards. The lighter coloured areas indicate the highest temperatures and the dark shows the lowest.
The device was tested on 12 people in a room maintained at 21.1 °C. The device was held in the mouth for 30 minutes with the oral cavity scanned just prior to insertion and immediately after it was removed.
As seen above, the Chemo Mouthpiece cools the entire oral cavity uniformly. Ice chips tend to only cool the bottom half of the mouth, making them less effective.
The results are certified by thermographer Julie Conner, CCT.
A Patient’s Journey
The value of the Chemo Mouthpiece for patients lies in its ability to minimise the worst of the symptoms from developing at all. Although it is only one part of what makes cancer so devastating, limiting the intensity of oral mucositis can bring huge benefits to patients’ quality of life: the infections, dehydration, malnutrition, and other conditions that can accompany oral mucositis are not only painful and expensive, but can also delay cancer treatments and ultimately hurt a patient’s overall prognosis. The good news is that avoiding these obstacles only takes one safe, simple, reusable device: the Chemo Mouthpiece.
The Chemo Mouthpiece Clinical Research Trial
Chemo Mouthpiece LLC. has engaged world renowned Oral Mucositis expert, Dr. Steve Sonis (currently with Harvard, Dana-Farber Cancer Institute and Brigham & Women’s Hospital), to lead our clinical research trial. This trial is assessing the efficacy and utility of our novel oral cryotherapy delivery device, the Chemo Mouthpiece™ (CMP™).
Specifically, we are assessing the ability of the CMP™ to mitigate symptoms associated with chemotherapy-induced oral mucositis in cancer patients being treated with standard mucotoxic chemotherapy regimens for many varied tumor types. This is a post-marketing, randomised, prospective, multi-site trial conducted within the United States. Ten top cancer institutes have been enlisted to participate in this clinical trial; these sites are located in California, Indiana, Missouri, Manhattan as well as New York State, Ohio, South Carolina, and Michigan.
The trial commenced in August 2020 with a planned accrual of 200 patients. These patients are randomised to either the Chemo Mouthpiece™ Arm or the Best Supportive Oral Care Arm.
The full list of participating sites as well as a synopsis of the clinical trial can be found at: https://clinicaltrials.gov/ct2/show/NCT04595838
